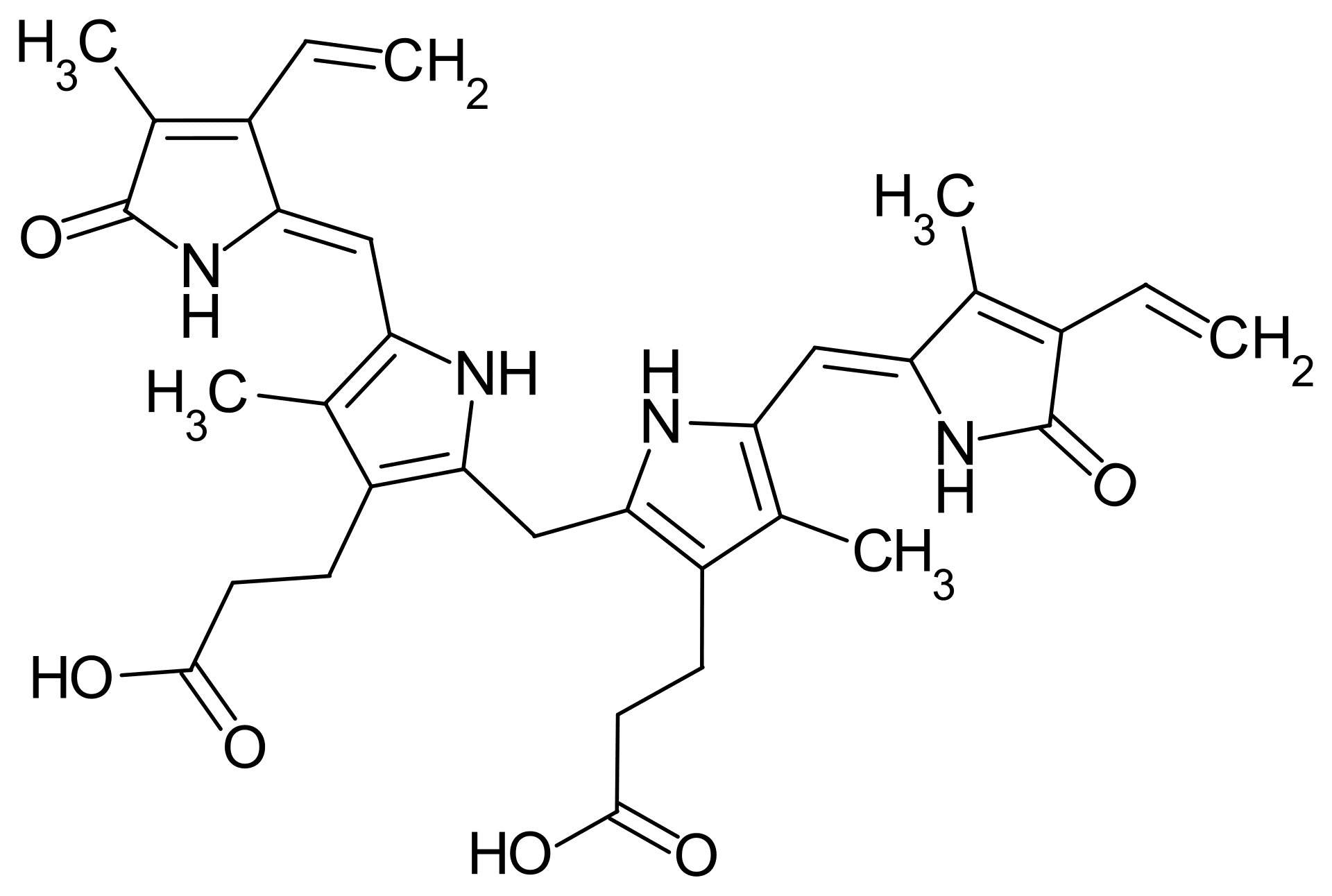
Serum Proteins and Vitamin A Transport
 Vitamin A is a fat-soluble vitamin essential for various biological functions and is transported in the bloodstream primarily bound to serum proteins. This binding is crucial for maintaining adequate vitamin A levels in the body and ensuring its delivery to target tissues. In this article, I’ll delve into the specific serum proteins involved in vitamin A transport, their mechanisms of binding, and the implications of these interactions for vitamin A metabolism and health.
Vitamin A is a fat-soluble vitamin essential for various biological functions and is transported in the bloodstream primarily bound to serum proteins. This binding is crucial for maintaining adequate vitamin A levels in the body and ensuring its delivery to target tissues. In this article, I’ll delve into the specific serum proteins involved in vitamin A transport, their mechanisms of binding, and the implications of these interactions for vitamin A metabolism and health.
Serum Proteins Involved in Vitamin A Transport
Several serum proteins contribute to the transport of vitamin A, each with its unique characteristics and functions. The two primary proteins involved are Retinol-Binding Protein (RBP) and Albumin.
RBP is the primary carrier of retinol (the alcohol form of vitamin A) in the bloodstream. RBP binds retinol with high affinity and specificity, forming a stable complex with transthyretin (TTR) to protect both proteins from degradation. This complex plays a crucial role in the absorption of vitamin A from the intestine and its distribution to various tissues, including the liver, adipose tissue, and retina.
Albumin is primarily a general carrier protein and can also bind retinol with lower affinity than RBP. This secondary role of albumin may be particularly important during periods of low RBP levels or increased retinol demand, serving as a reservoir for retinol.
Mechanisms of Vitamin A Binding to Serum Proteins
The binding of vitamin A to serum proteins is a complex process influenced by several factors. Hydrophobic interactions play a crucial role, as the nonpolar retinol molecule is attracted to the hydrophobic amino acid residues on the protein surface. This interaction helps to stabilize the complex. Additionally, hydrogen bonding between specific amino acids and the hydroxyl group of retinol contributes to the overall stability and specificity of the binding. The binding of retinol can also induce conformational changes in the protein, creating a more optimized binding site and enhancing the stability of the complex. These factors collectively contribute to the efficient and secure transport of vitamin A in the bloodstream.
Implications of Serum Protein-Vitamin A Interactions
The interaction between serum proteins and vitamin A has significant implications for vitamin A metabolism and health. The binding of vitamin A to serum proteins helps to maintain a stable concentration of the vitamin in the bloodstream, preventing excessive fluctuations that could lead to toxicity or deficiency. RBP-bound retinol is specifically targeted to cells and tissues that require vitamin A for their functions, such as the retina, epithelial cells, and immune cells. Additionally, the binding of retinol to serum proteins protects it from oxidative degradation and other metabolic processes. The levels of serum proteins involved in vitamin A transport can be influenced by various factors, including nutritional status, hormonal changes, and disease states. These factors can in turn affect vitamin A metabolism and bioavailability.
Closing Thoughts
The interaction between serum proteins and vitamin A is essential for maintaining adequate vitamin A levels in the body and ensuring its proper delivery to target tissues. RBP and albumin play key roles in this process, binding retinol with varying affinities and providing protection against degradation. Understanding these interactions is crucial for comprehending vitamin A metabolism, diagnosing vitamin A deficiency or toxicity, and developing therapeutic strategies for conditions related to vitamin A status.
Matthew A. Webster, MA, MS, ED.D, LPC
Dr. Matt Webster is a professional educator, nutritionist, and therapist located in the Houston, Texas area. He specializes in couples therapy, sexuality, and maladaptive eating patterns with a focus on the role of nutrition. More About Matt >>
Last modified: